Assertion A : Enol form of acetone [CH3COCH3] exists in <0.1% quantity. However, the enol
form of acetyl acetone [CH3COCH2OCCH3] exists in approximately 15% quantity.
Reason R : Enol form of acetylacetone is stabilized by intramolecular hydrogen bonding, which is
not possible in enol form of acetone.
Choose the correct statement:
Assertion A : Enol form of acetone [CH3COCH3] exists in <0.1% quantity. However, the enol
form of acetyl acetone [CH3COCH2OCCH3] exists in approximately 15% quantity.
Reason R : Enol form of acetylacetone is stabilized by intramolecular hydrogen bonding, which is
not possible in enol form of acetone.
Choose the correct statement:
The enol form of acetone exists in less than 0.1% quantity, since its keto form is highly stable. But in the case of acetylacetone, the enol form is stabilized by intramolecular H-bonding, so its quantity increases to approximately 15%.
The intramolecular H-bond in the enol form of acetylacetone is s
Similar Questions for you
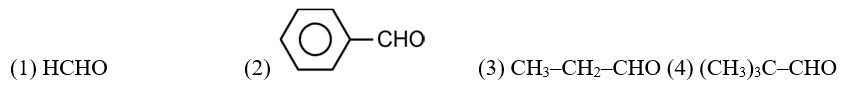
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering