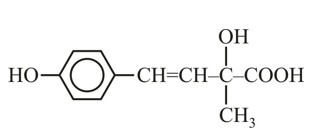
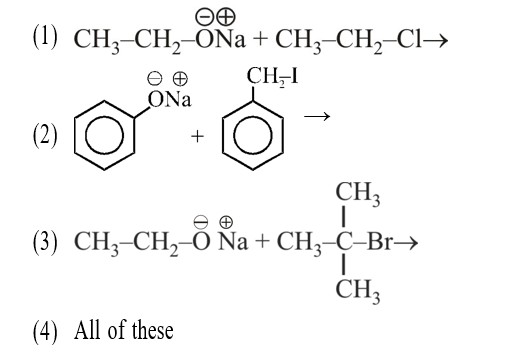
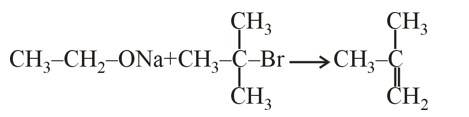
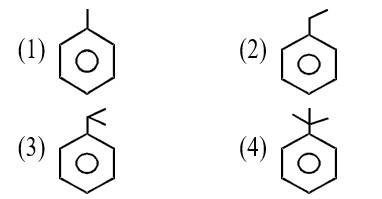
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: D
Because of the presence of electron withdrawing carbonyl groups, the alpha hydrogen atom in carbonyl compounds is acidic. In nature, hydrogen is very acidic.
Because the cation is released in the form of H+ , the anion
Similar Questions for you
ΔG° = –RT * 2.303 log K
–nFE° = +RT * 2.303 log K
2 * 96500 * 0.295 = 8.314 * 298 * 2.303 log10 K
10 = log10 K = 1010
It has chiral centre and differently di substituted double bonded carbon atoms.
Rate of ESR ∝ No. of α – H (Hyperconjugation)
Cr3+ion is a most stable in aqueous solution due to. t2g half filled configuration
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Twelve 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering