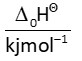At
and 1 atm pressure, the enthalpies of combustion are as given below:
Substance
H2
C(graphite
C2H6(g)
-286.0
-394.0
-1560.0
The enthalpy of formation of ethane is
At and 1 atm pressure, the enthalpies of combustion are as given below:
|
Substance |
H2 |
C(graphite |
C2H6(g) |
|
|
-286.0 |
-394.0 |
-1560.0 |
The enthalpy of formation of ethane is
Option 1 -
+54.0 kJ mol-1
Option 2 -
-68.0 kJ mol-1
Option 3 -
-86.0 kJ mol-1
Option 4 -
+97.0 kJ mol-1
-
1 Answer
-
Correct Option - 3
Detailed Solution:= (-788 – 858 + 1560) KJ/mole = -86 kJ/mole
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers