Atmospheric pressures recorded in different cities are as follows
Cities
p in N/m2
Shimla
1.01*105
Bangalore
1.2*105
Delhi
1.02*105
Mumbai
1.21*105
Consider the above data and mark the place at which liquid will boil first.
(i) Shimla
(ii) Bangalore
(iii) Delhi
(iv) Mumbai
Atmospheric pressures recorded in different cities are as follows
|
Cities |
p in N/m2 |
|
Shimla |
1.01*105 |
|
Bangalore |
1.2*105 |
|
Delhi |
1.02*105 |
|
Mumbai |
1.21*105 |
Consider the above data and mark the place at which liquid will boil first.
(i) Shimla
(ii) Bangalore
(iii) Delhi
(iv) Mumbai
This is a multiple choice answer as classified in NCERT Exemplar
option (i). Shimla
The temperature at which the liquid boils when the vapour pressure becomes equal to the atmospheric pressure is known as the boiling point of the liquid. When the atmospheric pressure is low, the boiling point of th
Similar Questions for you
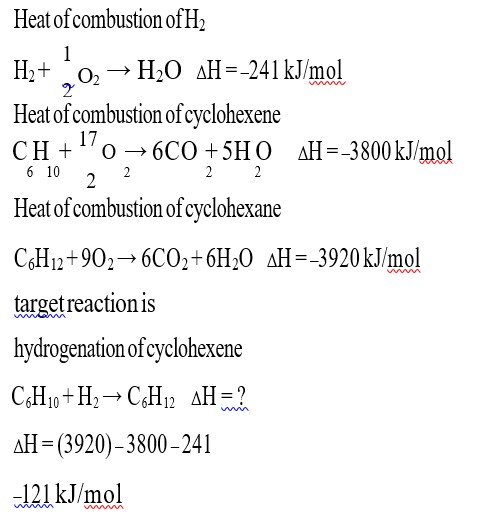
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Five 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering