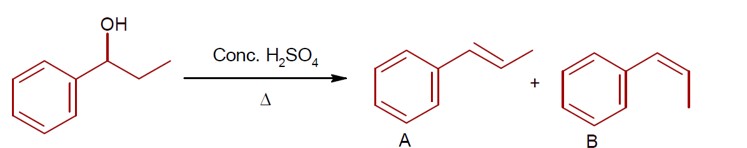
Consider the above reaction, and choose the correct statement:
Consider the above reaction, and choose the correct statement:
Option 1 - <p>Both compounds A and B are formed equally<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 2 - <p>The reaction is not possible in acidic medium</p>
Option 3 - <p>Compound A will be the major product<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 4 - <p>Compound B will be the major product</p>
1 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
A
Answered by
5 months ago
Correct Option - 1
Detailed Solution:
Trans product is more stable than Cis. ( Trans product feel less repulsion due to opposite direction)
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Eight 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering