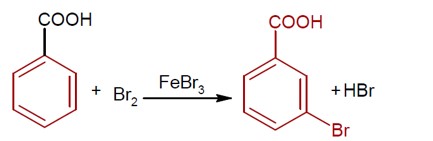
Consider the above reaction where 6.1 g of Benzoic acid is used to get 7.8g of m- bromo benzoic acid. The percentage yield of the product is ________. (Round off to the nearest integer).
[ Given : Atomic masses : C: 12.0u, H:1.0u, O:16.0u, Br:80.0 u]
Consider the above reaction where 6.1 g of Benzoic acid is used to get 7.8g of m- bromo benzoic acid. The percentage yield of the product is ________. (Round off to the nearest integer).
[ Given : Atomic masses : C: 12.0u, H:1.0u, O:16.0u, Br:80.0 u]
Moles of benzoic acid = 6.1 / 121 = 0.05
Theoretical moles of m- bromobenzoic acid = 0.05
Observed moles of m- bromobenzoic acid = 7.8 / 200 = 0.039
% yield = (0.039 / 0.05) * 100 = 78%
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering