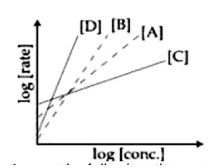
Consider the following reactions A→P1; B→P2; C→P3; D→P4.
The order of the above reactions are a,b,c and d, respectively. The following graph is obtained when log[rate] vs log[conc.] are plotted.
Among the following, the correct sequence for the order of the reactions is:
Consider the following reactions A→P1; B→P2; C→P3; D→P4.
The order of the above reactions are a,b,c and d, respectively. The following graph is obtained when log[rate] vs log[conc.] are plotted.
Among the following, the correct sequence for the order of the reactions is:
Option 1 -
d>a>b>c
Option 2 -
a>b>c>d
Option 3 -
c>a>b>d
Option 4 -
d>b>a>c
-
1 Answer
-
Correct Option - 4
Detailed Solution:rate=k [Conc]? log [rate]=logk+nlog [Conc]. 'n' is slope. Higher slope, higher order.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers