Discuss the nature of C—X bond in the haloarenes.
Discuss the nature of C—X bond in the haloarenes.
-
1 Answer
-
This is a short answer type question as classified in NCERT Exemplar
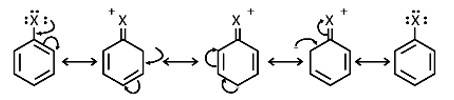
Resonance effect: Because the C – X bond has the characteristics of a partial double bond, it is extremely difficult to break.
(i) The C - X bond in haloarenes is extremely less reactive to nucleophilic groups
(ii) The C atom linked to the halogen is sp2 hybridised in the C – X bond. Carbon that has been sp2 hybridised with a higher s-character is more electronegative in nature and can retain the electron pair of the C - X bond more tightly than carbon that has been sp3 hybridised with a lower s -character in haloalkanes.
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers






