Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
-
1 Answer
-
This is a short answer type question as classified in NCERT Exemplar
In the presence of Lewis acid catalysts (iron or iron chloride), aryl bromides and chlorides can be made by electrophilic replacement of arenas with bromine and chlorine, respectively.
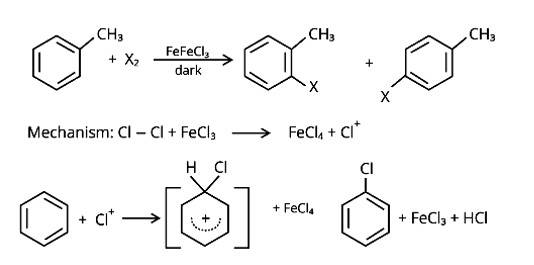
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers






