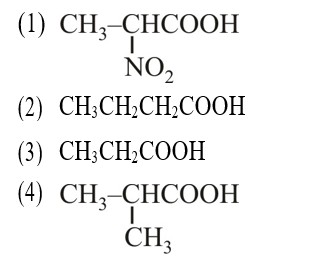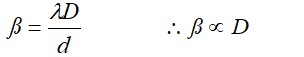For dry cleaning, in the place of tetrachloroethane, liquefied carbon dioxide with suitable detergent is an alternative solvent. What type of harm to the environment will be prevented by stopping use of tetrachloroethane? Will use of liquefied carbon dioxide with detergent be completely safe from the point of view of pollution? Explain.
For dry cleaning, in the place of tetrachloroethane, liquefied carbon dioxide with suitable detergent is an alternative solvent. What type of harm to the environment will be prevented by stopping use of tetrachloroethane? Will use of liquefied carbon dioxide with detergent be completely safe from the point of view of pollution? Explain.
-
1 Answer
-
This is a Long Answers Type Questions as classified in NCERT Exemplar
Tetrachloroethene (Cl2C=CCl2 )was earlier used as solvent for dry cleaning. This compound contaminates the groundwater and is also suspected carcinogen. The process using this compound is now being replaced by a process where liquefied CO2 is used. Replacement of halogenated solvent by liquid carbon dioxide will result in less harm to the groundwater. These days, hydrogen peroxide is used for the purpose of bleaching clothes in the process of laundry, which gives better results and makes use of a lesser amount of water.
Similar Questions for you
–I effect ∝ Acidic strength
+I effect ∝ Basic strength
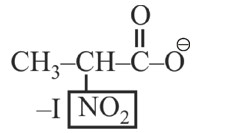
* Most stable anion due to maximum –I effect.
* Most acidic
with increase in separation of screen from slits plane, fringe width increases.
Excessive nitrate in drinking water causes methemoglobinemia
Excessive nitrate in drinking water causes methemoglobinemia
Release of toxic/undesirable materials in the environment.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers