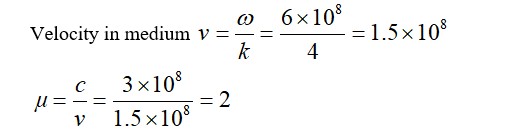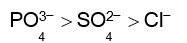For kinetic study of the reaction of iodide ion with H2O2 at room temperature:
(A) Always use freshly prepared starch solution.
(B) Always keep the concentration of sodium thiosulphate solution less than that of Kl solution.
(C) Record the time immediately after the appearance of blue colour.
(D) Record the time immediately before the appearance of blue colour.
(E) Always keep the concentration of sodium thiosulphate solution more than that of Kl solution.
Choose the correct answer from the options given below:
For kinetic study of the reaction of iodide ion with H2O2 at room temperature:
(A) Always use freshly prepared starch solution.
(B) Always keep the concentration of sodium thiosulphate solution less than that of Kl solution.
(C) Record the time immediately after the appearance of blue colour.
(D) Record the time immediately before the appearance of blue colour.
(E) Always keep the concentration of sodium thiosulphate solution more than that of Kl solution.
Choose the correct answer from the options given below:
Option 1 -
(A), (B), (C) only
Option 2 -
(A), (D), (E) only
Option 3 -
(D), (E) only
Option 4 -
(A), (B), (E) only
-
1 Answer
-
Correct Option - 1
Detailed Solution:Always fresh solution of starch used and recorded immediately after the blue colour appears. Na2S2O3 is used in limited amount.
Similar Questions for you
The process of settling of colloidal particles is
In physisorption multimolecular layers are formed on solid surface.
Emulsion is a colloidal solution of liquid in liquid.
Haemoglobin is a positive colloid. Hence greater is the charge of anion, more effective will be the coagulation of haemoglobin.
Therefore,
Correct order of coagulating power is
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers