For Rb(37) which of the following set of quantum numbers are correct for valence electron?
For Rb(37) which of the following set of quantum numbers are correct for valence electron?
Option 1 - <p><span lang="EN-IN">5, 0, 0, + <!-- [if gte mso 9]><xml>
<o:OLEObject Type="Embed" ProgID="Equation.DSMT4" ShapeID="_x0000_i1025"
DrawAspect="Content" ObjectID="_1820741411">
</o:OLEObject>
</xml><![endif]--></span><span class="mathml" contenteditable="false"> <math> <mrow> <mfrac> <mrow> <mn>1</mn> </mrow> <mrow> <mn>2</mn> </mrow> </mfrac> </mrow> </math> </span></p>
Option 2 - <p><span lang="EN-IN">5, 0, 1, –<span class="mathml" contenteditable="false"> <math> <mrow> <mfrac> <mrow> <mn>1</mn> </mrow> <mrow> <mn>2</mn> </mrow> </mfrac> </mrow> </math> </span> <!-- [if gte mso 9]><xml>
<o:OLEObject Type="Embed" ProgID="Equation.DSMT4" ShapeID="_x0000_i1025"
DrawAspect="Content" ObjectID="_1820741427">
</o:OLEObject>
</xml><![endif]--></span></p>
Option 3 - <p><span lang="EN-IN">5, 0, 1 + <!-- [if gte mso 9]><xml>
<o:OLEObject Type="Embed" ProgID="Equation.DSMT4" ShapeID="_x0000_i1025"
DrawAspect="Content" ObjectID="_1820741435">
</o:OLEObject>
</xml><![endif]--></span><span lang="EN-IN"><span contenteditable="false"> <math> <mrow> <mfrac> <mrow> <mn>1</mn> </mrow> <mrow> <mn>2</mn> </mrow> </mfrac> </mrow> </math> </span></span></p>
Option 4 - <p><span lang="EN-IN">5, 1, 1 + <!-- [if gte mso 9]><xml>
<o:OLEObject Type="Embed" ProgID="Equation.DSMT4" ShapeID="_x0000_i1025"
DrawAspect="Content" ObjectID="_1820741442">
</o:OLEObject>
</xml><![endif]--></span><span lang="EN-IN"><span contenteditable="false"> <math> <mrow> <mfrac> <mrow> <mn>1</mn> </mrow> <mrow> <mn>2</mn> </mrow> </mfrac> </mrow> </math> </span></span></p>
1 Views|Posted 4 months ago
Asked by Shiksha User
1 Answer
A
Answered by
4 months ago
Correct Option - 1
Detailed Solution:
37Rb = 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s1
Last electron enters in 5s subshell
Value of quantum numbers
n = 5, l = 0, m = 0, s =
Similar Questions for you
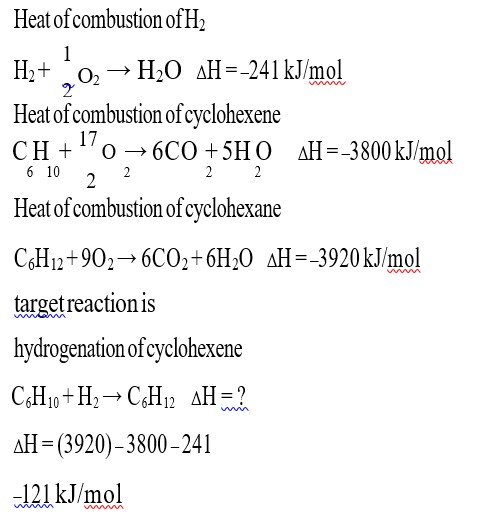
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering