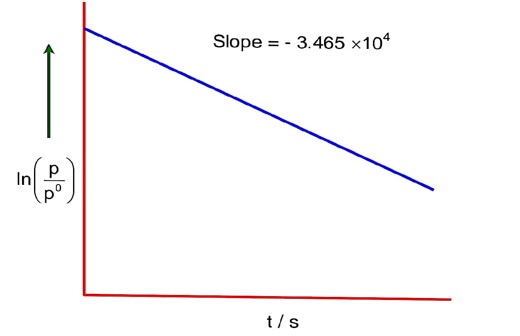
For the decomposition of azomethane,
CH3N2CH3(g) → CH3CH3(g) + N2(g), a first order reaction, the variation in partial pressure with time at 600 K is given as.
For the decomposition of azomethane,
CH3N2CH3(g) → CH3CH3(g) + N2(g), a first order reaction, the variation in partial pressure with time at 600 K is given as.
Asked by Shiksha User
-
1 Answer
-
For first order reaction,
sec
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers