Given below are the statements about diborane.
(A) Diborane is prepared by the oxidation of NaBH4 With I2.
(B) Each boron atom is in sp3 hybridized state.
(C) Diborane has one bridged 3 centre – 2 - electron bond.
(D) Diborane is a planar molecule.
The option with correct statement(s) is:
Given below are the statements about diborane.
(A) Diborane is prepared by the oxidation of NaBH4 With I2.
(B) Each boron atom is in sp3 hybridized state.
(C) Diborane has one bridged 3 centre – 2 - electron bond.
(D) Diborane is a planar molecule.
The option with correct statement(s) is:
Option 1 -
(A) only
Option 2 -
(C) only
Option 3 -
(A) and (B) only
Option 4 -
(C) and (D) only
-
1 Answer
-
Correct Option - 1
Detailed Solution:Diborane is produced when NaBH4 is reacted with I2
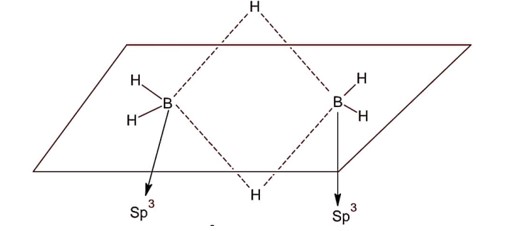 Both boron atoms are sp3 hybridised & molecule is non-planar. Diborane as two bridged 3 centre-2-electron bonds.
Both boron atoms are sp3 hybridised & molecule is non-planar. Diborane as two bridged 3 centre-2-electron bonds.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
