Given below are two statements one is labeled as Assertion A and the other is labeled as Reason R:
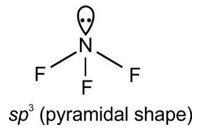
Assertion A: The amphoteric nature of water is explained by using Lewis acid/base concept
Reason R: Water acts as an acid with NH3 and as a base with H2S
In the light of the above statements choose the correct answer form the options given below:
Given below are two statements one is labeled as Assertion A and the other is labeled as Reason R:
Assertion A: The amphoteric nature of water is explained by using Lewis acid/base concept
Reason R: Water acts as an acid with NH3 and as a base with H2S
In the light of the above statements choose the correct answer form the options given below:
Option 1 -
Both A and R are true and R is the correct explanation of A.
Option 2 -
Both A and R are true but R is NOT the correct explanation of A.
Option 3 -
A is true but R is false.
Option 4 -
A is false but R is true.
-
1 Answer
-
Correct Option - 4
Detailed Solution:With H2S water acts like base and with NH3 it acts like acid.
Similar Questions for you
He2 has zero bond order hence it does not exist.
The three fundamental laws of chemistry are - Law of Definite Proportions, Law of Conservation of Mass, and Law of Multiple Proportions.
The three types of chemical bonds are - ionic, metallic and covalent bonds. When the electrons transfer between the atoms, they form the Ionic bonds by producing charged ions that are attracted to each other. When atoms share electrons, covalent bonds are created. When metal atoms share a sea of delocalized electrons, metallic bonds get created.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers