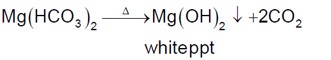Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: During the boiling of water having temporary hardness, Mg(HCO?)? is converted to MgCO?.
Reason R: The solubility product of Mg(OH)? is greater than that of MgCO?. In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: During the boiling of water having temporary hardness, Mg(HCO?)? is converted to MgCO?.
Reason R: The solubility product of Mg(OH)? is greater than that of MgCO?. In the light of the above statements, choose the most appropriate answer from the options given below:
Similar Questions for you
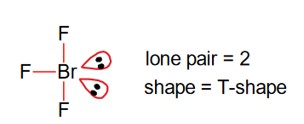
Hybridization of P in PF5 is sp3d, so value of y = 1
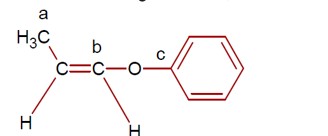
Hybridisation of carbon a, b, and c respectively are sp³, sp² and sp².
XeF? + SbF? → [XeF? ]? [SbF? ]?
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Chemical Bonding and Molecular Structure 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering