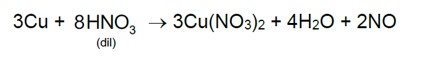Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): ICl is more reactive than I?.
Reason (R): I-Cl bond is weaker than I-I bond.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): ICl is more reactive than I?.
Reason (R): I-Cl bond is weaker than I-I bond.
In the light of the above statements, choose the most appropriate answer from the options given below:
I - Cl bond is stronger than I-I bond due to additional electrostatic force between I and Cl.
Similar Questions for you
HClO4 is the most acidic compound.
Group number = 11 (Atomic number = 111)
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
The inertness of
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering