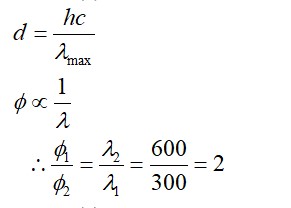Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R: The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below:
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R: The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below:
The para-magnetism of solution of alkali metals in liquid ammonia and its deep blue color is due to ammoniated electrons. Which absorb energy in visible region of light.
Similar Questions for you
Li+ has the highest hydration enthalpy.
Hence it is most hydrated
Therefore, Correct order of hydrated radii is Cs+ < Rb+ < K+ < Na+ < Li+
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering