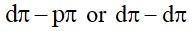Given below are two statements.
Statement I: During electrolytic refining, blister copper deposits precious metals.
Statement II: In the process of obtaining pure copper by electrolysis method, copper blister is used to make the anode.
In the light of the above statements, choose the correct answer form the option given below:
Given below are two statements.
Statement I: During electrolytic refining, blister copper deposits precious metals.
Statement II: In the process of obtaining pure copper by electrolysis method, copper blister is used to make the anode.
In the light of the above statements, choose the correct answer form the option given below:
Option 1 -
Both Statement I and Statement II are true.
Option 2 -
Both Statement I and Statement II are false.
Option 3 -
Statement I is true but Statement II is false.
Option 4 -
Statement I is false but Statement II is true.
-
1 Answer
-
Correct Option - 1
Detailed Solution:In electro-refining, impure metal (blister copper) is used as an anode while precious metal like Au, Pt gets deposited as anode mud.
Similar Questions for you
Na+ C + N + S ®NaSCN
Fe3+ + SCN–
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
Ellingham diagram explains the feasibility of reduction process not the kinetics of process.
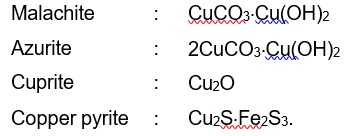
Malachite : CuCO3.Cu (OH)2
Azurite : 2CuCO3.Cu (OH)2
Cuprite : Cu2O
Copper pyrite : Cu2S.Fe2S3.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers