Given below are two statements:
Statement I : The nucleophilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
Statement II : The nucleophillic addition of hydrogen cyanide to an aldehyde or a ketone yields amine as final product.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements:
Statement I : The nucleophilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
Statement II : The nucleophillic addition of hydrogen cyanide to an aldehyde or a ketone yields amine as final product.
In the light of the above statements, choose the most appropriate answer from the options given below:
Option 1 -
Statement I is true but Statement II is false.
Option 2 -
Both Statement I and Statement II are true
Option 3 -
Both Statement I and Statement II are false.
Option 4 -
Statement I is false but Statement II is true
-
1 Answer
-
Correct Option - 1
Detailed Solution:Nucleophilic addition of sodium hydrogen sulphite to aldehyde or ketone is a;
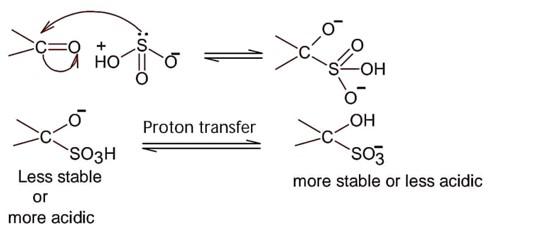
So; nucleopilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
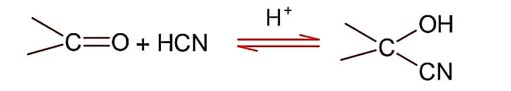
Addition of hydrogen cyanide;
Final product is cyanohydrin.
Similar Questions for you
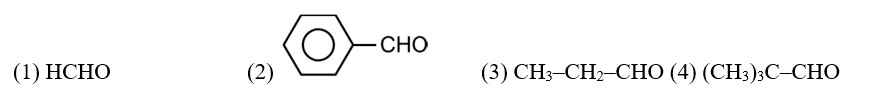
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers