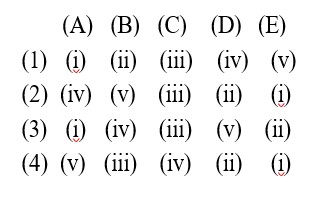
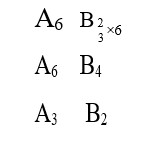How would you expect the bond strength to change in the series C2 to C2 to C-22 ?
How would you expect the bond strength to change in the series C2 to C2 to C-22 ?
Option 1 - <p><span lang="EN-IN">Gets stronger than weaker </span></p>
Option 2 - <p><span lang="EN-IN">Gets Weaker</span></p>
Option 3 - <p><span lang="EN-IN">Gets stronger </span></p>
Option 4 - <p><span lang="EN-IN">Gets weaker than stronger</span></p>
6 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
V
Answered by
5 months ago
Correct Option - 3
Detailed Solution:
Kindly consider the following figure
As bond order is increasing it implies bond strength is increasing. As bond order is increasing it implies bond strength is increasing.
Similar Questions for you
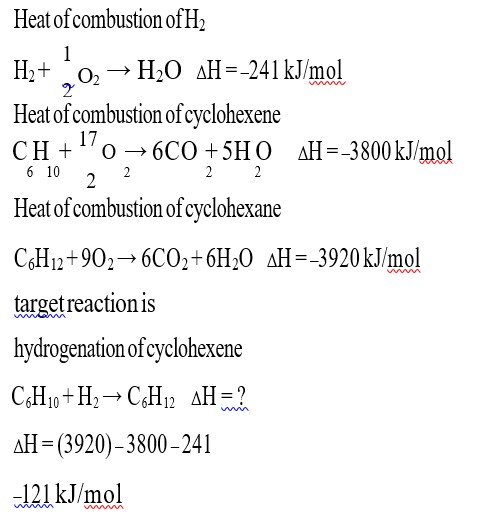
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Chemical Bonding and Molecular Structure 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering