Identify the incorrect statement for PCl5 from the following
Identify the incorrect statement for PCl5 from the following
Option 1 -
In this molecule, orbital of phosphorous are assumed to undergo sp3d hybridization
Option 2 -
The geometry of PCl5 is trigonal bipyramidal
Option 3 -
PCl5 has axial bonds stronger than three equatiorial bons
Option 4 -
The three equatiorial bonds of PCl5 lie in a plane
-
1 Answer
-
Correct Option - 3
Detailed Solution:Structure PCl5 is trigonal bipyramidal,
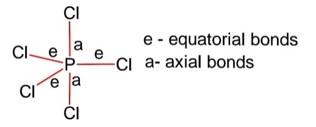
Hybridization of P is sp3d
Equatorial bonds lie in a plane
Axial bonds are longer than equatorial bonds so axial bonds are weaker than equatorial bonds.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
