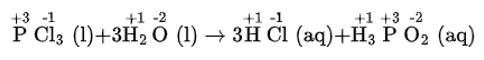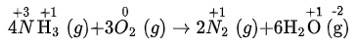Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
(i) 3HCl(aq) + HNO3 (aq) → Cl2 (g) + NOCl (g) + 2H2O (l )
(ii) HgCl2 (aq) + 2KI (aq) → HgI2 (s) + 2KCl (aq)
(iii) Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
(iv) PCl3 (l) + 3H2O (l) → 3HCl (aq) + H3 PO3 (aq)
(v) 4NH3 + 3O2 (g) → 2N2 (g) + 6H2O (g)
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
(i) 3HCl(aq) + HNO3 (aq) → Cl2 (g) + NOCl (g) + 2H2O (l )
(ii) HgCl2 (aq) + 2KI (aq) → HgI2 (s) + 2KCl (aq)
(iii) Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
(iv) PCl3 (l) + 3H2O (l) → 3HCl (aq) + H3 PO3 (aq)
(v) 4NH3 + 3O2 (g) → 2N2 (g) + 6H2O (g)
-
1 Answer
-
This is a Short answer type question as classified in NCERT Exemplar
(i) We can write the given reaction along with their oxidation numbers as-
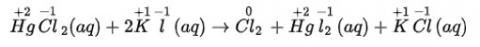
As, chlorine is oxidised in hydrochloric acid (behaving as reducing agent) -as its oxidation number is increases during the reaction from -1 to 0) and nitric acid is reduced (behaving as oxidising agent) as its oxidation number is decreases during the reaction from +5 to +3). Hence, it is the redox reaction.
(ii) We can write the given reaction along with their oxidation numbers as-
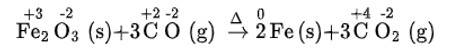
Here, oxidation number of none of the atoms change hence it is not a redox reaction.
(iii) We can write
...more
Similar Questions for you
Kindly go through the solution
(c) Li
Kindly go through the solution
(c) Al
Kindly go through the solution
(d) +6
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers