(ii) Reaction of liquid hydrazine (N2H4 ) with chlorate ion (ClO3– ) in basic medium produces nitric oxide gas and chloride ion in gaseous state. (Balance by oxidation number method)
(ii) Reaction of liquid hydrazine (N2H4 ) with chlorate ion (ClO3– ) in basic medium produces nitric oxide gas and chloride ion in gaseous state. (Balance by oxidation number method)
-
1 Answer
-
This is a Short answer type question as classified in NCERT Exemplar
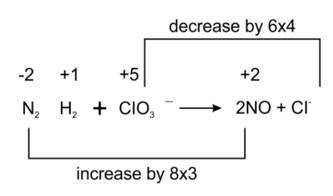
We can balance the given reaction by oxidation number method-
Balancing by oxidation number method as to make the electron gain and loss equal as given
3N2H4 + 4ClO3- ? 6NO + 4Cl- + 6H2O
The balanced chemical is given as-
Similar Questions for you
Kindly go through the solution
(c) Li
Kindly go through the solution
(c) Al
Kindly go through the solution
(d) +6
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers
