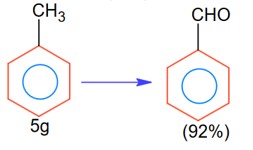
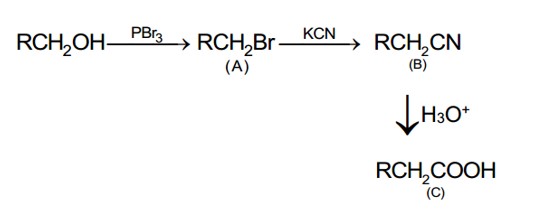
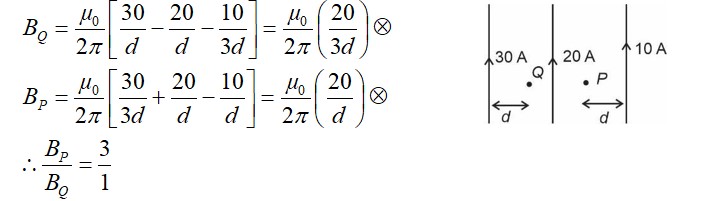In the below reaction, 5g of toluene is converted into benzaldehyde with 92% yield. The amount of benzaldehyde produced is____________× 10-2 g. (Nearest integer)
In the below reaction, 5g of toluene is converted into benzaldehyde with 92% yield. The amount of benzaldehyde produced is____________× 10-2 g. (Nearest integer)
-
1 Answer
-
No. of moles =
Moles of benzaldehyde =
Mass of benzaldehyde = 5 × 10-2 ×106 = 5.3 gm
= 530 × 10-2 gm
Similar Questions for you
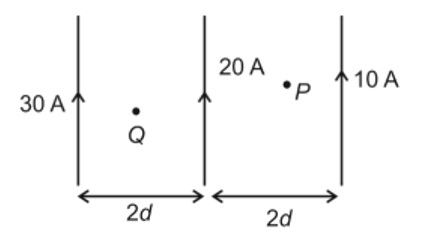
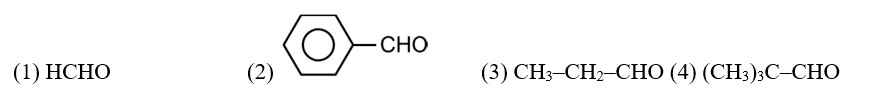
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers