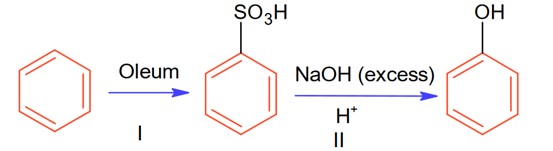
In the following reaction
The % yield for reaction I is 60% and that of reaction II is 50%. The overall yield of the complete reaction is___________% [Nearest integer]
In the following reaction
The % yield for reaction I is 60% and that of reaction II is 50%. The overall yield of the complete reaction is___________% [Nearest integer]
-
1 Answer
-
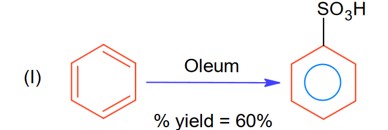
Let initial moles of reactant taken = n. Total moles obtained for benzene sulphonic acid = 0.6 n (with % yield = 60%)
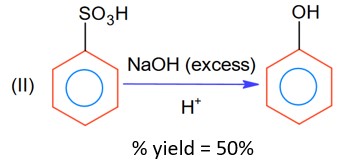
Moles of benzene sulphonic acid before reaction II = 0.6n
Moles obtained for phenol (with % yield 50%) = 0.6 × 0.5 n = 0.3 n
So, overall % age yield of complete reaction =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers