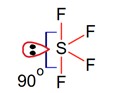
In the structure of SF4, the lone pair of electrons on S is in.
In the structure of SF4, the lone pair of electrons on S is in.
Option 1 -
Equatorial position and there are two lone pair – bond pair repulsions at 90°.
Option 2 -
Equatorial position and there are three lone pair – bond pair repulsions at 90°.
Option 3 -
Axial position and there are three lone – bond pair repulsion at 90°.
Option 4 -
Axial position and there are two lone – bond pair repulsion at 90°.
-
1 Answer
-
Correct Option - 1
Detailed Solution:Geometry of SF4 is trigonal bipyramidal, in which there is one lonepair which occupy equatorial position as,
There are two lone pair – bond pair repulsions at 90°
Similar Questions for you
s – block and p – block elements together are called as representative elements.
Zn (Z = 30) is a d-block element.
Kindly go through the answer
Element/ion | |
O | -141 |
Na | -53 |
Cl | -349 |
O- | +780 |
You can read the classification of elements and periodicity in properties class 11 notes to study class 11 ch3 and score well in class 11th exams and NEET, and JEE exams.
When the elements having similar properties repeat at regular intervals, it is called periodicity in the properties of elements.
Classification of Elements is Necessary because it helps in understanding and remembering elements' properties better, you can understand how elements react or behave, and can guess properties of unidentified or newly discovered elements.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers