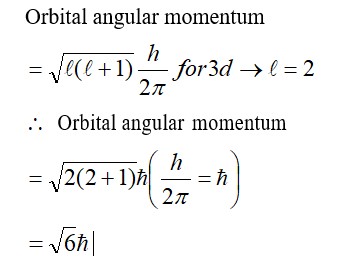
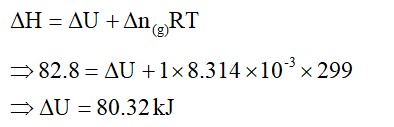Increase in enthalpy of the surroundings is equal to decrease in enthalpy of the system. Will the temperature of system and surroundings be the same when they are in thermal equilibrium?
Increase in enthalpy of the surroundings is equal to decrease in enthalpy of the system. Will the temperature of system and surroundings be the same when they are in thermal equilibrium?
-
1 Answer
-
This is a Short Answer Type Questions as classified in NCERT Exemplar
Yes, when the system and the surroundings are in thermal equilibrium, their temperatures are the same.
Similar Questions for you
Kindly go through the solution
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers