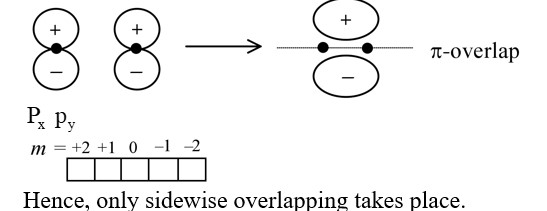
Main axis of a diatomic molecule is  , molecular orbital px and py overlap to form which of the following orbitals.
, molecular orbital px and py overlap to form which of the following orbitals.
Main axis of a diatomic molecule is ![]() , molecular orbital px and py overlap to form which of the following orbitals.
, molecular orbital px and py overlap to form which of the following orbitals.
For overlap, the lobes of the atomic orbitals are perpendicular to the line joining the nuclei.
Similar Questions for you
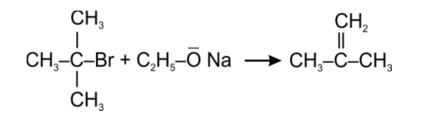
Tertiary haloalkane does not undergo SN2 reaction
Bond strength Bond order
NO ® Number of electron = 7 + 8 = 15
B.O. Similar to
B.O. of N2 = 3 B.O of C2 =
Removal of e- form antibonding molecular orbital increases bond order.
In NO & O2 has valance e- in p orbital
O? (15) will have configuration σ1s²σ1s²σ2s²σ2s²σ2p? ² (π2p? ²=π2p? ²) (π*2p? ¹). This ion is paramagnetic.
B.O. of CO = 3
B.O. of NO? = 3
Both are isoelectronic
So difference = 0
∴ x = 0
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Chemical Bonding and Molecular Structure 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering