Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.
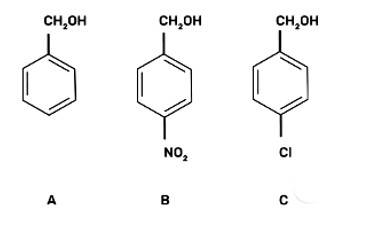
Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.

I. a < b < c
II. b < a < c
III. b < c < a
IV. c < b < a
5 Views|Posted 7 months ago
Asked by Shiksha User
1 Answer
P
Answered by
7 months ago
This is a multiple choice answer as classified in NCERT Exemplar
(III) b < c < a
The reaction will proceed by SN1 mechanism to form the carbocation intermediate, more the stability of carbocation more will be the reactivity of the given compound with HBr/HC1.
-NO2 group is an electron withdrawing group and d
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.8L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering







