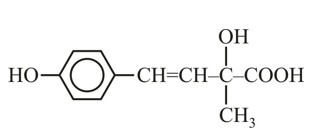
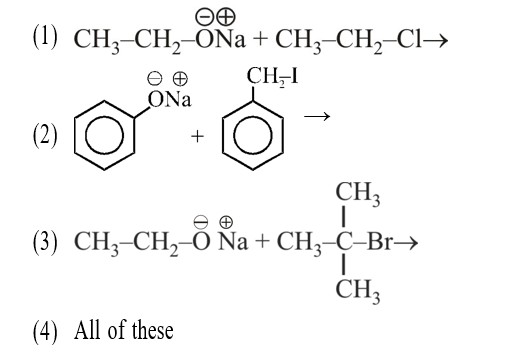
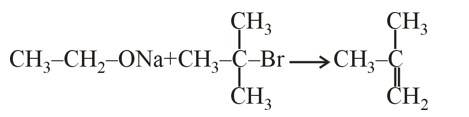
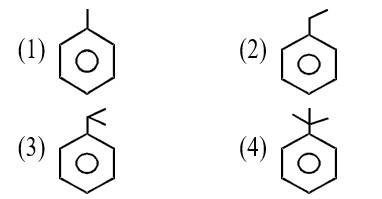
Match List-I with List-II.
List – I List – II
(Parameter) (Unit)
(A) Cell constant (i) S cm2 mol-1
(B) Molar conductivity (ii) Dimensionless
(C) Conductivity (iii) m-1
(D) Degree of dissociation of electrolyte (iv) Ω-1 m-1
Choose the most appropriate answer from the options given below:
Match List-I with List-II.
List – I List – II
(Parameter) (Unit)
(A) Cell constant (i) S cm2 mol-1
(B) Molar conductivity (ii) Dimensionless
(C) Conductivity (iii) m-1
(D) Degree of dissociation of electrolyte (iv) Ω-1 m-1
Choose the most appropriate answer from the options given below:
(a) Cell constant =
(b) molar conductivity
= Scm2 mol-1
(c) Conductivity
(d) degree of dissociation =
Similar Questions for you
ΔG° = –RT * 2.303 log K
–nFE° = +RT * 2.303 log K
2 * 96500 * 0.295 = 8.314 * 298 * 2.303 log10 K
10 = log10 K = 1010
It has chiral centre and differently di substituted double bonded carbon atoms.
Rate of ESR ∝ No. of α – H (Hyperconjugation)
Cr3+ion is a most stable in aqueous solution due to. t2g half filled configuration
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Electrochemistry 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering