Match the complex species given in Column I with the possible isomerism given in Column II and assign the correct code
Column I (Complex species)
Column II (Isomerism)
A. [Co(NH3)4Cl2]+
1. Optical
B. cis - [Co(en)2Cl2]+
2. Ionisation
C. [Co(NH3)5(NO2)]Cl2
3. Coordination
D. [Co(NH3)6][Cr(CN)6]
4. geometrical
5. linkage
Code :
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (4) B (1) C (5) D (3)
(iv) A (4) B (1) C (2) D (3)
Match the complex species given in Column I with the possible isomerism given in Column II and assign the correct code
|
Column I (Complex species) |
Column II (Isomerism) |
|
A. [Co(NH3)4Cl2]+ |
1. Optical |
|
B. cis - [Co(en)2Cl2]+ |
2. Ionisation |
|
C. [Co(NH3)5(NO2)]Cl2 |
3. Coordination |
|
D. [Co(NH3)6][Cr(CN)6] |
4. geometrical |
|
5. linkage |
Code :
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (4) B (1) C (5) D (3)
(iv) A (4) B (1) C (2) D (3)
-
1 Answer
-
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: Correct option: (iv)
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
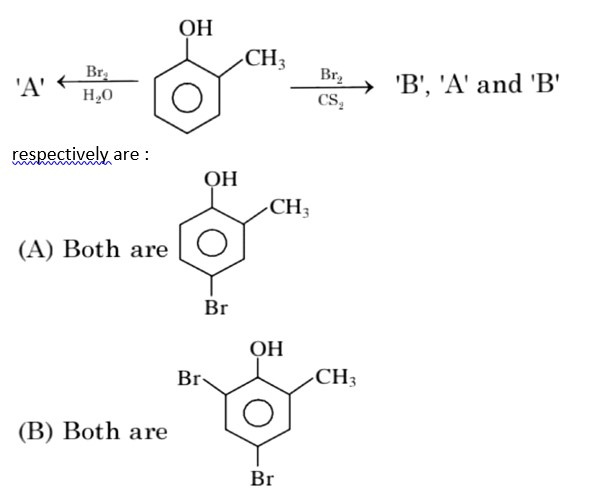
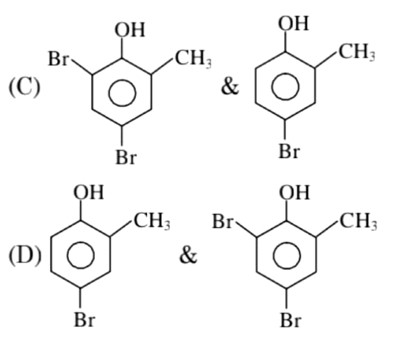
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers