Methylation of 10g of benzene give 9.2g of toluene. Calculate the percentage yield of toluene_________. (Nearest initeger)
Methylation of 10g of benzene give 9.2g of toluene. Calculate the percentage yield of toluene_________. (Nearest initeger)
Asked by Shiksha User
-
1 Answer
-
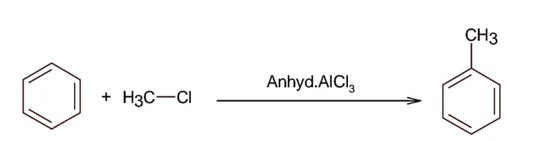
Theoretically; 1 mol benzene gives 1 mol toluene
Moles of benzene =
Moles of toluene (Theoretical) =
Mole of toluene (observed) =
% yield =
= 78%
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
