Name the type of isomerism when ambidentate ligands are attached to central metal ion. Give two examples of ambidentate ligands.
Name the type of isomerism when ambidentate ligands are attached to central metal ion. Give two examples of ambidentate ligands.
-
1 Answer
-
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Ambidentate ligands are ligands which have two donating sites. Coordinating compounds containing ambidentate ligands show linkage isomerism due to two different binding positions. Linkage isomerism have same ligand and geometry attached to a central metal ion by different donating sites
Examples:
(i)
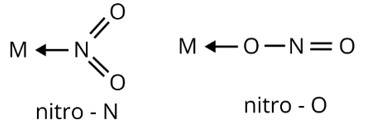
(ii) M←SCN
thiocyanato
M←NCS
isothiocyanato
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
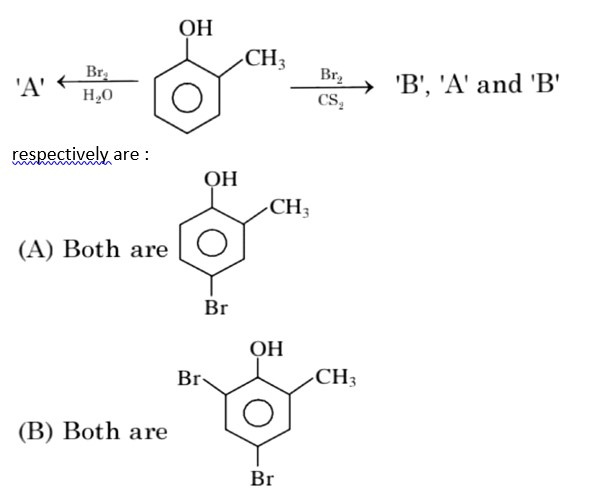
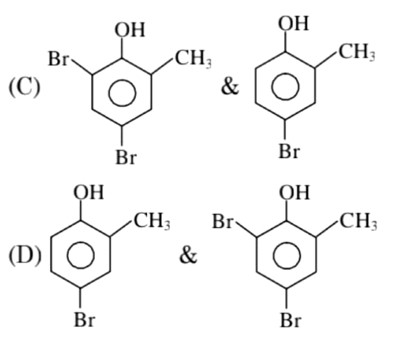
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers