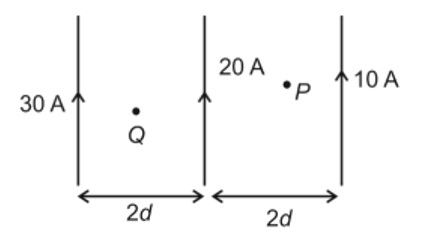
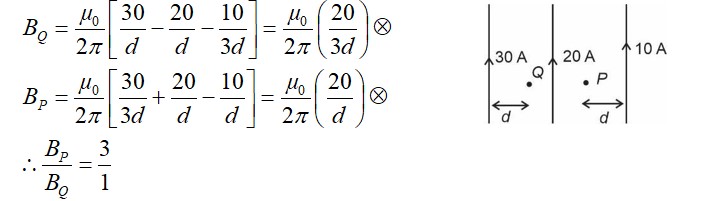Number of paramagnetic oxides among the following given oxides is_________.
Li2O, CaO, Na2O2, KO2, MgO and K2O
Number of paramagnetic oxides among the following given oxides is_________.
Li2O, CaO, Na2O2, KO2, MgO and K2O
Option 1 -
0
Option 2 -
1
Option 3 -
2
Option 4 -
3
-
1 Answer
-
Correct Option - 2
Detailed Solution:Given are the oxide of alkali and alkaline earth metals which are ionic in nature.
Simple oxide are Li2O, CaO, MgO and K2O.
Peroxide is Na2O2 and superoxide is KO2.
All simple oxides are diamagnetic as it has no unpaired electron.
Similar Questions for you
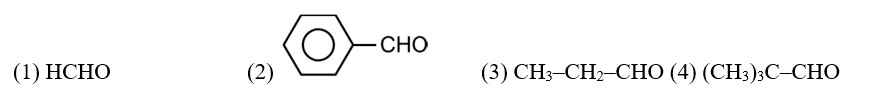
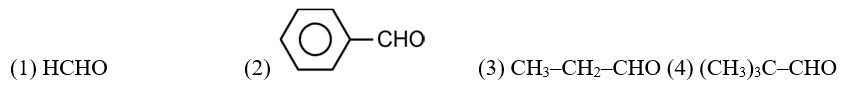
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers