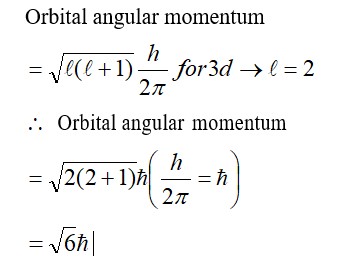
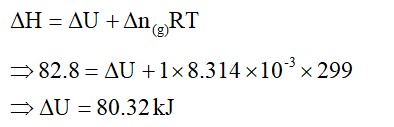One mole of acetone requires less heat to vapourise than 1 mol of water. Which of the two liquids has higher enthalpy of vapourisation?
One mole of acetone requires less heat to vapourise than 1 mol of water. Which of the two liquids has higher enthalpy of vapourisation?
-
1 Answer
-
This is a Short Answer Type Questions as classified in NCERT Exemplar
Due to weak force of attraction between molecules, acetone requires less heat to vaporise. Hence, water has higher enthalpy of vaporization.
Similar Questions for you
Kindly go through the solution
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers