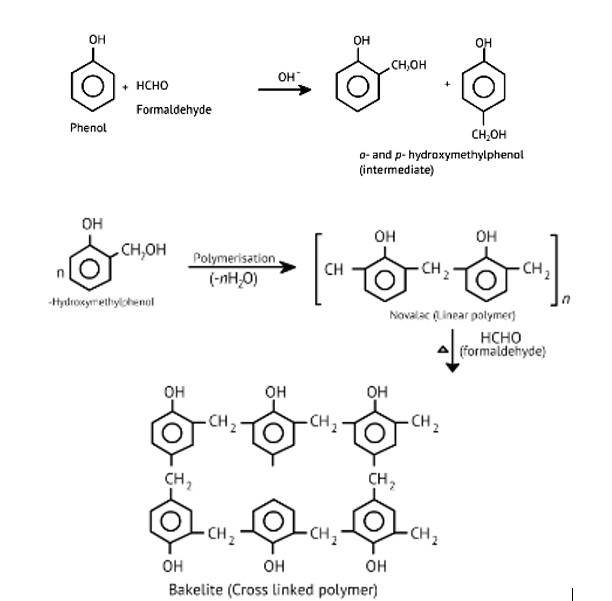
Phenol and formaldehyde undergo condensation to give a polymer (A) which on heating with formaldehyde gives a thermosetting polymer (B). Name the polymers. Write the reactions involved in the formation of (A). What is the structural difference between two polymers?
Phenol and formaldehyde undergo condensation to give a polymer (A) which on heating with formaldehyde gives a thermosetting polymer (B). Name the polymers. Write the reactions involved in the formation of (A). What is the structural difference between two polymers?
-
1 Answer
-
This is a Long Answer Type Questions as classified in NCERT Exemplar
The oldest synthetic polymers are phenol-formaldehyde polymers. These are made by combining phenol and formaldehyde in the presence of either an acid or a base catalyst. The reaction begins with the formation of o-and/or p hydroxymethyl phenol derivatives, which then react with phenol to form compounds with shaving rings connected by –CH2 groups. The first product could be a linear product, such as Novolac.
When heated with formaldehyde, Novolac crosslinks to form Bakelite, an infusible solid mass.
It is used to make combs, phonograph records, electrical switches,
...more
Similar Questions for you
Kindly go through the solution
CH2 = CHCN (acrylonitrile) is a monomer of orlon
Monomer of neoprene polymer is
3-Chloro-1, 3-butadiene
CH2 = CHCN (acrylonitrile) is a monomer of orlon
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers