Predict the product of reaction of aniline with bromine in non-polar solvent such as CS2.
Predict the product of reaction of aniline with bromine in non-polar solvent such as CS2.
-
1 Answer
-
This is a Short Type Questions as classified in NCERT Exemplar
Ans: CS2 is a non-polar solvent which decreases the activating effect of? NH2. As a result, mono substitution occurs only at o- and p- positions giving a mixture of 2-bromoaniline (minor) and 4-bromoaniline (major) as products.
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
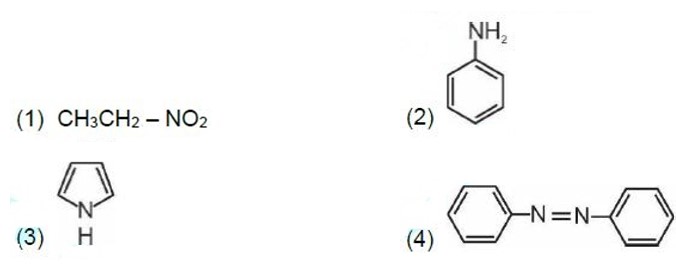
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
Correct order of basic strength in aqueous medium is
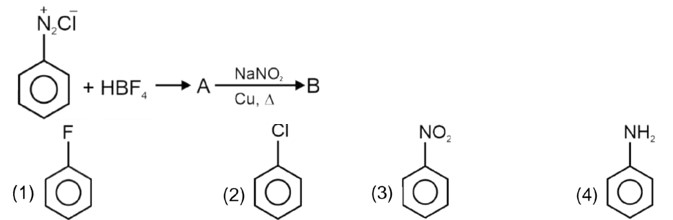
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers