Quantum number for outermost electron of K-atom are given by
Quantum number for outermost electron of K-atom are given by
Option 1 - <p><span lang="EN-IN">n = 4, l = 0, m = 0, <!-- [if gte mso 9]><xml>
<o:OLEObject Type="Embed" ProgID="Equation.DSMT4" ShapeID="_x0000_i1025"
DrawAspect="Content" ObjectID="_1820234467">
</o:OLEObject>
</xml><![endif]--> </span><span class="mathml" contenteditable="false"> <math> <mrow> <mi>s</mi> <mo>=</mo> <mfrac> <mrow> <mn>1</mn> </mrow> <mrow> <mn>2</mn> </mrow> </mfrac> </mrow> </math> </span></p>
Option 2 - <p><span lang="EN-IN">n = 4, l = 1, m = 0, <!-- [if gte mso 9]><xml>
<o:OLEObject Type="Embed" ProgID="Equation.DSMT4" ShapeID="_x0000_i1025"
DrawAspect="Content" ObjectID="_1820234473">
</o:OLEObject>
</xml><![endif]--> <span class="mathml" contenteditable="false"> <math> <mrow> <mi>s</mi> <mo>=</mo> <mfrac> <mrow> <mn>1</mn> </mrow> <mrow> <mn>2</mn> </mrow> </mfrac> </mrow> </math> </span></span></p>
Option 3 - <p><span lang="EN-IN">n = 3, l = 0, m = 0, <!-- [if gte mso 9]><xml>
<o:OLEObject Type="Embed" ProgID="Equation.DSMT4" ShapeID="_x0000_i1025"
DrawAspect="Content" ObjectID="_1820234481">
</o:OLEObject>
</xml><![endif]--></span><span class="mathml" contenteditable="false"> <math> <mrow> <mi>s</mi> <mo>=</mo> <mfrac> <mrow> <mn>1</mn> </mrow> <mrow> <mn>2</mn> </mrow> </mfrac> </mrow> </math> </span></p>
Option 4 - <p><span lang="EN-IN">n = 4, l = 0, m = 1,<span class="mathml" contenteditable="false"> <math> <mrow> <mi>s</mi> <mo>=</mo> <mfrac> <mrow> <mn>1</mn> </mrow> <mrow> <mn>2</mn> </mrow> </mfrac> </mrow> </math> </span> <!-- [if gte mso 9]><xml>
<o:OLEObject Type="Embed" ProgID="Equation.DSMT4" ShapeID="_x0000_i1025"
DrawAspect="Content" ObjectID="_1820234491">
</o:OLEObject>
</xml><![endif]--></span></p>
2 Views|Posted 4 months ago
Asked by Shiksha User
1 Answer
A
Answered by
4 months ago
Correct Option - 1
Detailed Solution:
K19 = 1s22s22p63s23p64s1
For 4s electron
n = 4
l = 0
m = 0
Similar Questions for you
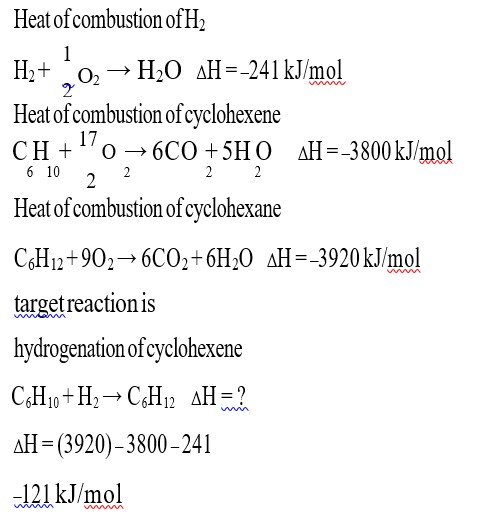
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering