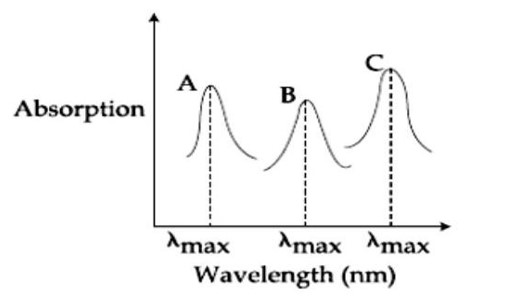
Simplified absorption spectra of three complexes ((i), (ii) and (iii)) of Mⁿ⁺ ion are provided below; their λ_max values are marked as A, B and C respectively. The correct match between the complexes and their λ_max values is:
(i) [M(NCS)₆]⁽⁻⁶⁺ⁿ⁾
(ii) [MF₆]⁽⁻⁶⁺ⁿ⁾
(iii) [M(NH₃)₆]ⁿ⁺
Simplified absorption spectra of three complexes ((i), (ii) and (iii)) of Mⁿ⁺ ion are provided below; their λ_max values are marked as A, B and C respectively. The correct match between the complexes and their λ_max values is:
(i) [M(NCS)₆]⁽⁻⁶⁺ⁿ⁾
(ii) [MF₆]⁽⁻⁶⁺ⁿ⁾
(iii) [M(NH₃)₆]ⁿ⁺
Option 1 -
A-(i), B-(ii), C-(iii)
Option 2 -
A-(ii), B-(iii), C-(i)
Option 3 -
A-(iii), B-(i), C-(ii)
Option 4 -
A-(ii), B-(i), C-(iii)
-
1 Answer
-
Correct Option - 3
Detailed Solution:Ligand field strength: NH? > NCS? > F? Stronger ligand, higher Δ, lower λ_max.
So λ (NH? ) < λ (NCS? ) < λ (F? ). A=λ (F? ), B=λ (NCS? ), C=λ (NH? ). A-ii, B-i, C-iii.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers