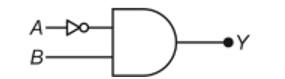
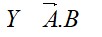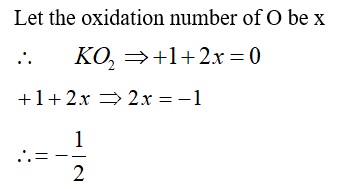Taking stability as the factor, which one of the following represents correct relationship?
Taking stability as the factor, which one of the following represents correct relationship?
Option 1 - <p>TlI > TlI₃<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 2 - <p>TlCl₃ > TlCl<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 3 - <p>InI₃ > InI<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 4 - <p>AlCl > AlCl₃</p>
2 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
V
Answered by
5 months ago
Correct Option - 1
Detailed Solution:
Tl? ³ is less stable than Tl? ¹ (inert pair effect). Going down the group 13, stability of lower oxidation state increases. In case of B, Al, Ga and In, higher O.S. +3 remains more stable than lower O.S. +1 . But, in last stable element, thallium (Tl), lower O.S. +1 become more stable than higher O.
Similar Questions for you
From BF3 to BI3 Lewis acidic strength increases
F2 is the strongest oxidising agent
HClO4 is the most acidic compound.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering