Tert-Butyl Bromide reacts with aq. NaOH by SN1 mechanism while n-butyl bromide reacts by SN2 mechanism. Why?
Tert-Butyl Bromide reacts with aq. NaOH by SN1 mechanism while n-butyl bromide reacts by SN2 mechanism. Why?
-
1 Answer
-
This is a short answer type question as classified in NCERT Exemplar
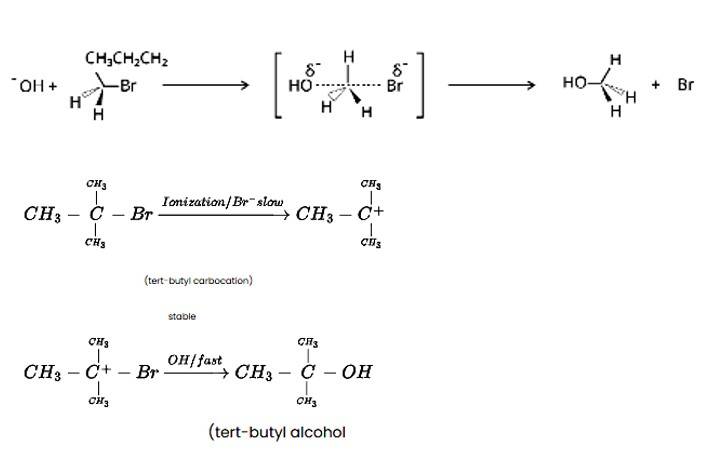
Tert-butyl bromide is substituted via the SN1 process because it may produce a stable carbocation in the first step after the halide group is cleaved. The nucleophile OH - interacts with the carbocation next. The primary halide n-butylbromide, on the other hand, is unable to create a stable carbocation, thus it undergoes the SN2 process, which is a one-step substitution involving OH - attack and concomitant X - leaving to generate n-butyl alcohol.
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers






