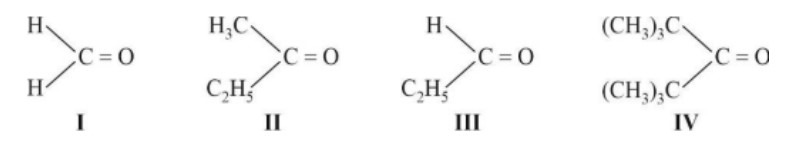
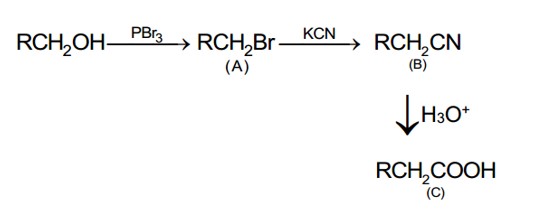
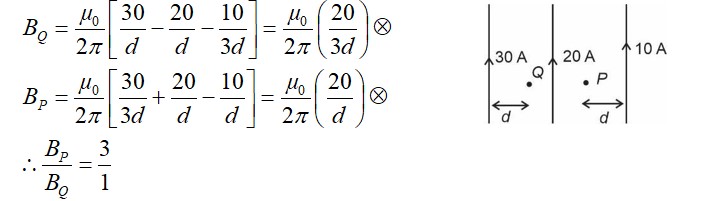The correct order of reactivity for the nucleophilic addition reaction of the following carbonyl compounds with ethyl magnesium iodide is:
The correct order of reactivity for the nucleophilic addition reaction of the following carbonyl compounds with ethyl magnesium iodide is:
If the carbonyl compound is sterically crowded, then it will be reluctant to undergo addition reaction. Moreover, attachment of bulkier alkyl group with the carbonyl carbon decreases the partial positive charge resulting into the minimization of attack by R? from RMgBr. So, the order is
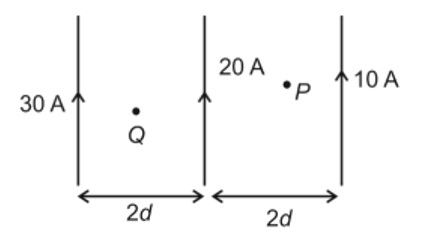
Similar Questions for you
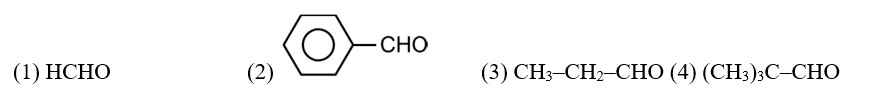
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering