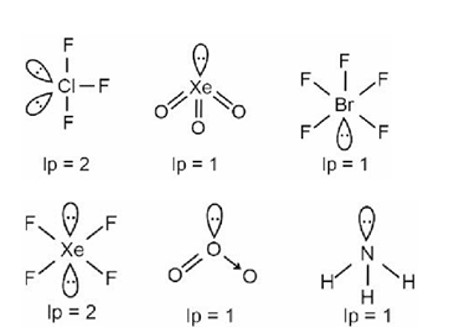
The correct shape and I – I – I bond angles respectively in ion are:
The correct shape and I – I – I bond angles respectively in ion are:
Option 1 -
Linear; 180°
Option 2 -
T – shaped ; 180° and 90°
Option 3 -
Trigonal planar; 120°
Option 4 -
Distorted trigonal planar; 135° and 90°
-
1 Answer
-
Correct Option - 1
Detailed Solution:Kindly consider the following figure
Linear (180°)
Similar Questions for you
PF5, PCl5, PBr5, Fe (CO)5 Þ Trigonal bipyramidal
BrF5 Þ Square pyramidal
[PtCl4]2– Þ Square planar
SF6 Þ Octahedral
During the electrolysis of dilute H2SO4
In the solid form of dihedral angle is equal to 90.2°.
Total number of electron in Ti = 22
Total number of electron in Ti? = 22 – 4 = 18 So EAN value of Ti = 18 + 12 + 4 = 34
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers