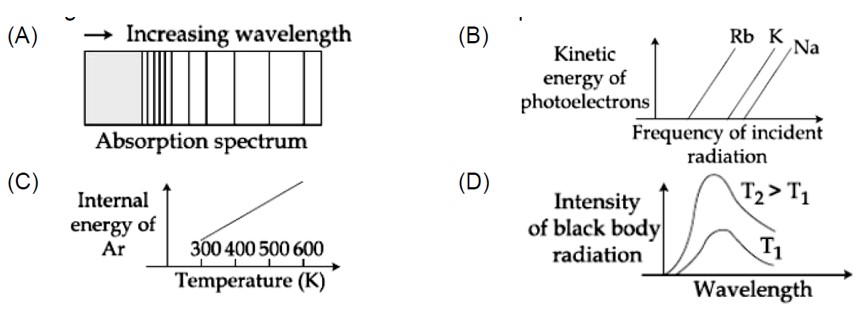
The figure that is not a direct manifestation of the quantum nature of atom is
The figure that is not a direct manifestation of the quantum nature of atom is
Option 1 -
a
Option 2 -
b
Option 3 -
c
Option 4 -
d
-
1 Answer
-
Correct Option - 3
Detailed Solution:1, 2 and 3 are according to quantum theory but (4) is statement of kinetic theory of gases
Similar Questions for you
Through Heisenberg's Uncertainty Principle, it's proven that you can't pin down where an electron is and how fast it's moving at the same time. The Bohr model did not look into this. The main reason for that thought was that it pictured electrons like little planets that would move in a loop around the nucleus. But that only works if you know both position and speed exactly. Nature doesn't let you do that. Add to it the fact that electrons also behave like waves, and the Bohr model just couldn't keep up. That's why scientists had to move on to the quantum model, which fits way better with how electrons actually act.
De Broglie had the idea that everything that moves has a bit of wave behaviour. Technically, that includes any person, a football, and even a bus. The catch is that for big things, the mass is so large that their wavelength is insanely tiny. It's so tiny it's impossible to notice. That's why you don't see a football spreading out like ripples when you kick it. Electrons though? They're super light, so their wavelengths are big enough for us to actually measure. And when scientists did experiments, such as electron diffraction, the electrons really did behave like waves. That was the proof De Broglie needed to show he was ri
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers