The following solutions were prepared by dissolving 10 g of glucose (C₆H₁₂O₆) in 250ml of water (P₁), 10 g of urea (CH₄N₂O) in 250ml of water (P₂) and 10 g of sucrose (C₁₂H₂₂O₁₁) in 250ml of water (P₃). The right option for the decreasing order of osmotic pressure of these solutions is :
The following solutions were prepared by dissolving 10 g of glucose (C₆H₁₂O₆) in 250ml of water (P₁), 10 g of urea (CH₄N₂O) in 250ml of water (P₂) and 10 g of sucrose (C₁₂H₂₂O₁₁) in 250ml of water (P₃). The right option for the decreasing order of osmotic pressure of these solutions is :
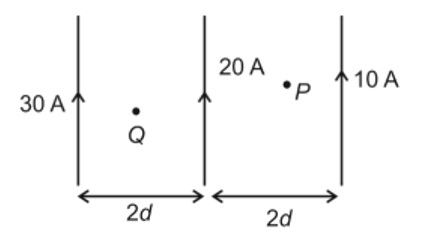
π = iCRT
P? = 1 * (10/180) * R * T (For Glucose)
P? = 1 * (10/60) * R * T (For Urea)
P? = 1 * (10/342) * R * T (For Sucrose)
∴ P? > P? > P?
Similar Questions for you
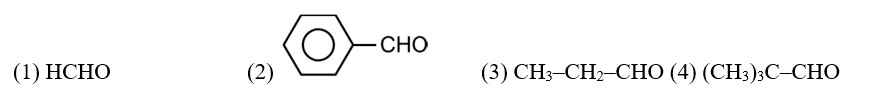
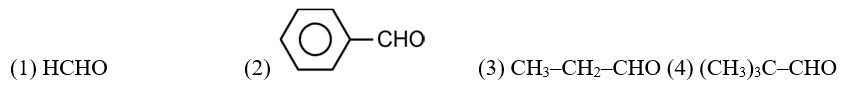
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering