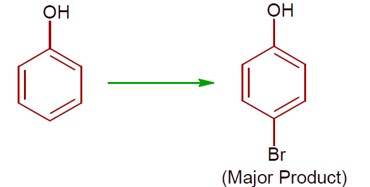
The given reaction can occur in the presence of:
(a) Bromine water
(b) Br? in CS?, 273 K
(c) Br? / FeBr?
(d) Br? in CHCl?, 273 K
Choose the correct answer from the options given below:
The given reaction can occur in the presence of:
(a) Bromine water
(b) Br? in CS?, 273 K
(c) Br? / FeBr?
(d) Br? in CHCl?, 273 K
Choose the correct answer from the options given below:
Option 1 -
(a) and (c) only
Option 2 -
(b), (c) and (d) only
Option 3 -
(b) and (d) only
Option 4 -
(a), (b) and (d) only
-
1 Answer
-
Correct Option - 4
Detailed Solution:(a) Phenol with Br? / H? O gives 2,4,6-tribromophenol. (High ionisation due to polar solvent and high activation of ring)
(b) Phenol with Br? in CS? /CHCl? /FeBr? gives a mixture of o- and p-bromophenol, with p-bromophenol as the major product. (less ionisation due to non- polar solvent)
[Chemical reactions showing bromination of phenol under different conditions]
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers