The ionization enthalpy of Na+ formation from Na(g) is 495.8 kJ mol-1, while the electron gain enthalpy of Br is - 325.0 kJ mol-1. Given the lattice enthalpy of NaBr is - 728.4 kJ mol-1. The energy for the formation of NaBr ionic solid is (-) -------------- × 10-1kJ mol-1.
The ionization enthalpy of Na+ formation from Na(g) is 495.8 kJ mol-1, while the electron gain enthalpy of Br is - 325.0 kJ mol-1. Given the lattice enthalpy of NaBr is - 728.4 kJ mol-1. The energy for the formation of NaBr ionic solid is (-) -------------- × 10-1kJ mol-1.
-
1 Answer
-
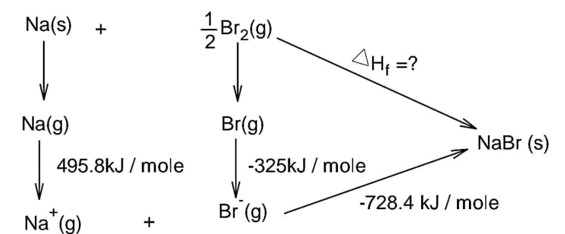
Bond dissociation energy of Br2 = 228 kJ/mole
Note : In question is neglect
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
