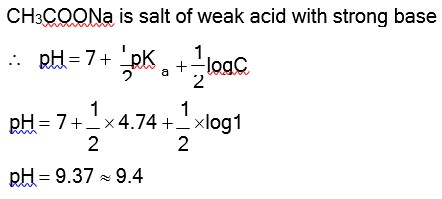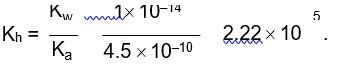The pH of 1 M CH3COONa solution in water will be nearly (pKa for CH3COOH = 4.74)
The pH of 1 M CH3COONa solution in water will be nearly (pKa for CH3COOH = 4.74)
Option 1 - <p>6.4</p>
Option 2 - <p>10.4</p>
Option 3 - <p>9.4</p>
Option 4 - <p>7.4</p>
17 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
A
Answered by
5 months ago
Correct Option - 3
Detailed Solution:
Similar Questions for you
0.01 M NaOH,
M = 1 * 10-2
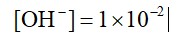
pOH = 2
pH = 2
Kp = Kc (RT)Dng
36 * 10–2 = Kc (0.0821 * 300)–1
Kc = 0.36 * 0.0821 * 300 = 8.86 » 9
A(g) ->B(g) + (g)
Initial moles n 0 &nbs
On increasing pressure, equilibrium moves in that direction where number of gaseous moles decreases.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Chemical Equilibrium 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering