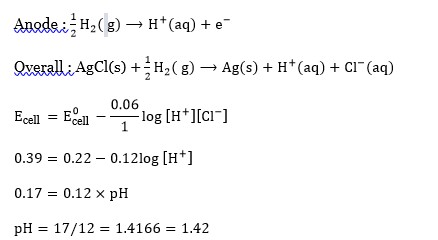The photoelectric current from Na (work function, w? = 2.3eV) is stopped by the output voltage of the cell
Pt(s)/H?(g, 1 bar) | HCl (aq., pH = 1)|AgCl(s)|Ag(s).
The pH of aq. HCl required to stop the photoelectric current from K(w? = 2.25 eV), all other conditions remaining the same, is × 10?² (to the nearest integer).
Given,
2.303(RT/F) = 0.06 V; E°(AgCl|Ag|Cl?) = 0.22 V
The photoelectric current from Na (work function, w? = 2.3eV) is stopped by the output voltage of the cell
Pt(s)/H?(g, 1 bar) | HCl (aq., pH = 1)|AgCl(s)|Ag(s).
The pH of aq. HCl required to stop the photoelectric current from K(w? = 2.25 eV), all other conditions remaining the same, is × 10?² (to the nearest integer).
Given,
2.303(RT/F) = 0.06 V; E°(AgCl|Ag|Cl?) = 0.22 V
-
1 Answer
-
Kindly consider the following Image
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers